AI-Powered Regulatory Assistance for Pharmasift
Pharmasift, a leader in pharmaceutical regulatory compliance, simplifies access to Office of Prescription Drug Promotion (OPDP) enforcement action letters, CFR guidelines, and regulatory documents. However, manual review of promotional materials and regulatory research was time-consuming and inefficient. To address these challenges, Code District developed a Generative AI-powered chatbot and automated review system, significantly improving efficiency, accuracy, and compliance workflows.
80% reduction in document review time
90% faster regulatory search

New Jersey, USA
Pharmaceutical
October 2023 - Present
Services Used
“Working with Code District was an incredible experience. Their team didn’t just build a chatbot for us—they completely transformed the way we interact with regulatory data. The AI-powered search and analysis tool has drastically cut down our review times from weeks to minutes, making compliance checks far more efficient. What stood out the most was their ability to truly understand our business needs and proactively find solutions that made an impact. Their expertise in AI and their commitment to delivering a high-quality product made all the difference.”

CEO, Pharmasift
The Challenge
Pharmasift faced several operational bottlenecks that hindered efficiency and compliance accuracy:
-
Manual Review Delays
Reviewing pharmaceutical promotional materials against regulatory guidelines often took weeks, slowing down approval cycles and increasing operational overhead.
-
Inefficient Research Process
Compliance teams had to manually search through large databases to find relevant CFR guidelines and enforcement letters, making it difficult to extract the right information quickly.
-
Scalability Issues
As regulations evolved and the volume of enforcement letters increased, Pharmasift struggled to efficiently analyze compliance risks and deliver timely insights to its users.
-
Inconsistent Compliance Feedback
The manual review process left room for human errors and inconsistencies, leading to potential compliance risks.
Our Solution
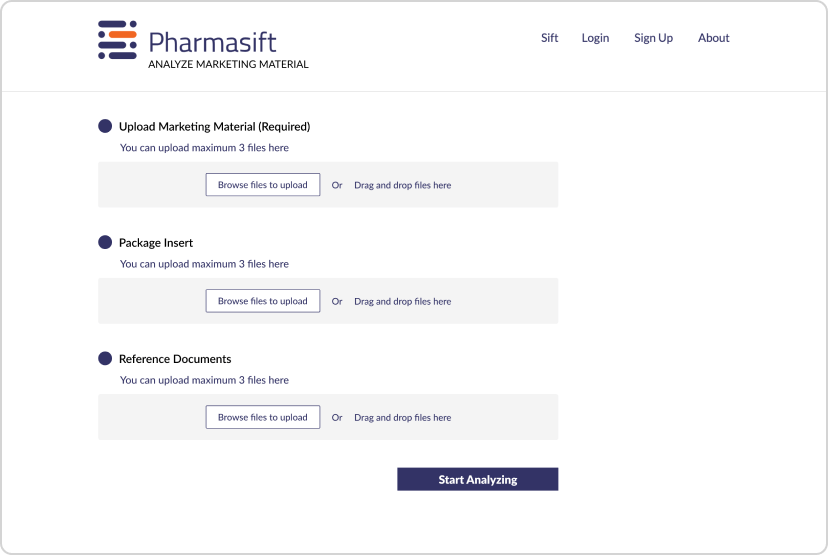
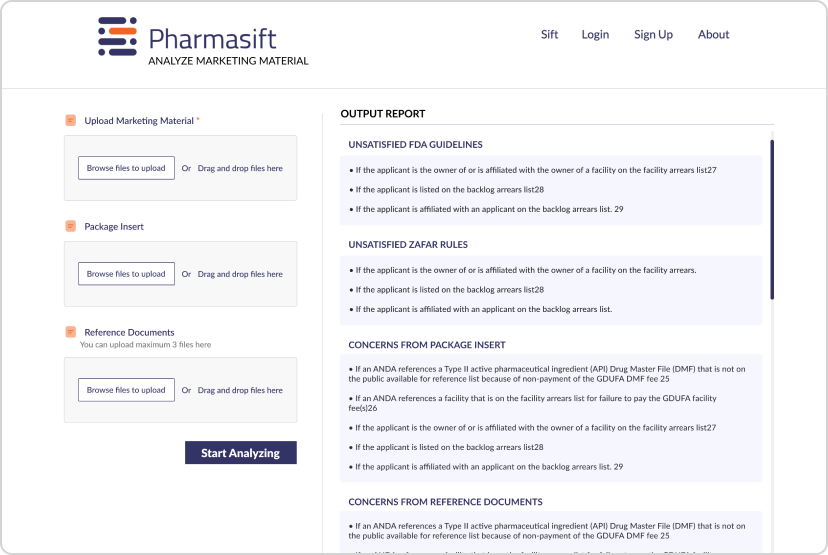
To solve these challenges, we developed two AI-powered tools: Pharmasift Insights for automated compliance review and Pharmasift Chat for instant regulatory search. These solutions leveraged Generative AI, Retrieval-Augmented Generation (RAG), and hybrid search models to optimize compliance workflows and enable faster, more accurate decision-making.
Pharmasift Insights – AI-Powered Compliance Review
To accelerate promotional material review, we developed Pharmasift Insights, an AI-powered system that analyzes documents in minutes instead of weeks.
Automated Document Analysis
The system reviews promotional materials based on CFR guidelines and enforcement history, ensuring compliance with regulatory standards.
AI-Driven Feedback & References
It provides detailed feedback on non-compliant content and references past enforcement actions to guide compliance improvements.
Streamlined Analyst Workflow
Instead of manually reviewing every document, analysts can quickly validate AI-generated feedback, reducing time spent on each review.
Pharmasift Chat – Instant Regulatory Search
To simplify regulatory research, we built Pharmasift Chat, an AI-powered chatbot that enables users to ask compliance-related questions and instantly retrieve relevant guidelines, letters, and references.
Hybrid Search Model
Uses both structured SQL queries for database searches and semantic search for retrieving relevant text from vector databases.
LLM-Powered Query Understanding
Analyzes user input, selects the best retrieval method, and delivers accurate, contextual responses.
Retrieval-Augmented Generation (RAG)
Ensures responses are backed by authoritative data, preventing AI hallucinations.
The Results
reduction in document review time, cutting compliance analysis from 2 weeks to 5 minutes.
faster regulatory search, delivering relevant results in under 3 seconds.
improvement in accuracy, minimizing compliance errors with AI-driven analysis.
increase in analyst productivity, reducing time spent on manual research.
scalability boost, efficiently handling growing regulatory data without added workload.
consistency in compliance decisions by standardizing rule enforcement across all promotional materials
Technology Stack
Share your business goals with technical experts
Sales and general inquires
sales@codedistrict.comCall us
+1 (703) 940-1971“Code District successfully launched our application on time. The team worked hard, adjusted to our schedule, and ensured our requests were turned around very quickly. They asked the right questions, used sound judgment, and made consistent progress, demonstrating strong technical skills and a driven attitude.”
